Companion Diagnostics Market Market Entry Strategies and Forecast to 2033
 Kiran Aggarwal
21 Sep, 2025
7 mins read
23
Kiran Aggarwal
21 Sep, 2025
7 mins read
23
Companion Diagnostics Market Definition & Importance
Companion diagnostics (CDx) are specialized medical tests designed to identify whether a patient will benefit from a specific therapeutic product or face risks associated with its use. They play a critical role in precision medicine by linking diagnostic testing with targeted treatment, ensuring more effective, personalized care. CDx technologies are particularly vital in oncology, but their applications are expanding into autoimmune, cardiovascular, central nervous system (CNS), and infectious diseases. By enabling healthcare providers to tailor therapies, companion diagnostics help improve treatment efficacy, reduce adverse effects, and optimize healthcare costs.
Market Size & Growth Rate
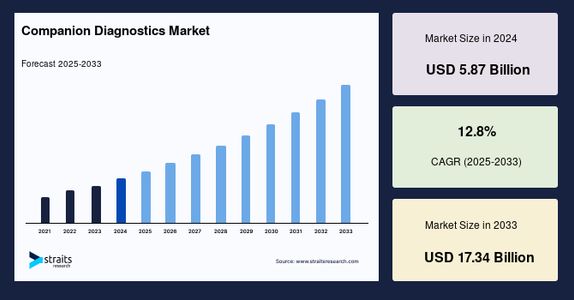
According to Straits Research, the global companion diagnostics market size was valued at USD 5.87 billion in 2024 and is projected to grow from USD 6.62 billion in 2025 to USD 17.34 billion by 2033, at a strong CAGR of 12.8% during the forecast period (2025–2033).
Key Drivers of Growth
The companion diagnostics market is expanding due to several critical factors:
- Rising adoption of precision medicine: Increasing demand for targeted therapies, particularly in oncology, is accelerating CDx integration into treatment pathways.
- Advancements in genomics and molecular diagnostics: Innovations in PCR, next-generation sequencing (NGS), and immunohistochemistry have enhanced the accuracy, speed, and cost-effectiveness of CDx tests.
- Growing cancer burden worldwide: The surge in cancer incidence is driving demand for diagnostic solutions that can optimize therapeutic efficacy.
- Regulatory support and co-development models: Agencies like the FDA are increasingly approving drugs alongside companion diagnostics, streamlining their adoption.
- Pharma–diagnostic collaborations: Partnerships between pharmaceutical companies and diagnostic developers are driving innovation pipelines and accelerating market penetration.
Key Competitors in the Companion Diagnostics Market
- Abbott
- Agilent Technologies
- Hoffmann-La Roche Ltd
- Biomerieux SA
- Qiagen NV
- Almac Group
- Danaher Corporation
- Illumina, Inc.
- Myriad Genetics, Inc.
- Sysmex Corporation
- Thermo Fisher Scientific Inc.
- Abnova Corporation
- Guardant Health, Inc.
Request Sample @ https://straitsresearch.com/report/companion-diagnostics-market/request-sample
Market Segmentation
By Technology
- Polymerase Chain Reaction (PCR)
- Next-generation Sequencing (NGS)
- Immunohistochemistry
- In Situ Hybridization
- Others
By Indication
- Oncology
- Autoimmune Diseases
- Cardiovascular Diseases
- Central Nervous System Disorders
- Virology Diseases
- Others
Key Trends & Innovations
- Shift toward liquid biopsies: Non-invasive blood-based tests are gaining traction as alternatives to tissue biopsies.
- Integration of AI and bioinformatics: Artificial intelligence and machine learning are enhancing the predictive power of CDx by analyzing large genomic datasets.
- Companion diagnostics beyond oncology: Expanding into autoimmune and cardiovascular conditions as precision medicine broadens its scope.
- Partnership-driven growth: Pharma companies are co-developing drugs with diagnostics to reduce risks and accelerate approvals.
- Point-of-care (POC) advancements: Development of rapid CDx tests is improving accessibility and turnaround times in clinical settings.
Regional Insights & Market Share
- North America: Dominates the global market due to advanced healthcare infrastructure, high adoption of personalized medicine, and strong regulatory frameworks.
- Europe: Significant market share, supported by favorable reimbursement policies and research-driven growth in precision medicine.
- Asia-Pacific: Fastest-growing region, driven by rising cancer prevalence, expanding healthcare access, and growing clinical trial activities in China, India, and Japan.
- Latin America & Middle East & Africa: Emerging markets with improving diagnostic infrastructure and government-led healthcare initiatives, though adoption remains slower compared to developed regions.
Challenges/Restraints
- High costs of advanced diagnostic tests limiting adoption in price-sensitive markets.
- Complex regulatory requirements for co-development of drugs and diagnostics.
- Limited awareness among clinicians in developing regions.
- Reimbursement hurdles affecting patient access in certain healthcare systems.
Get Detailed Segmentation @ https://straitsresearch.com/report/companion-diagnostics-market/segmentation
Future Outlook & Strategic Implications
The companion diagnostics market is set for rapid expansion, fueled by its pivotal role in precision medicine. Strategic collaborations between pharma and diagnostic players will remain central to accelerating new product development. Future growth will be supported by the adoption of liquid biopsy, AI-driven testing platforms, and broader disease coverage beyond oncology. Companies focusing on affordability and regulatory alignment will have the edge in emerging markets, while innovations in genomic data integration and POC testing will drive accessibility worldwide.
About StraitsResearch
StraitsResearch delivers industry-leading analysis and intelligence across multiple sectors, empowering businesses, investors, and strategists with the data they need to make informed decisions. Our team of analysts combines rigorous research methodologies with deep market understanding to provide actionable insights and forecasts.
Contact:
- Website: https://straitsresearch.com/
- Email: sales@straitsresearch.com
- Address: Siddhi Tower, Office C & D, 4th Floor, Pune
Written By:
Kiran Aggarwal
Hotels at your convenience
Now choose your stay according to your preference. From finding a place for your dream destination or a mere weekend getaway to business accommodations or brief stay, we have got you covered. Explore hotels as per your mood.